|
Shares of Rockwell Medical, Inc. (NASDAQ:RMTI) Drops by -8.7% - Insider Trading Report |
 |
 |
|
Rockwell Medical, Inc. (NASDAQ:RMTI) has lost 8.7% during the past week and dropped 23.86% in the last 4 weeks. The shares are however, negative as compared to the S&P 500 for the past week with a loss of 3.11%. Rockwell Medical, Inc. (NASDAQ:RMTI) has underperformed the index by 19.66% in the last 4 weeks. Investors should watch out for further signals and trade with caution.
For the current week, the company shares have a recommendation consensus of Buy. Rockwell Medical, Inc. (NASDAQ:RMTI) rose 4.52% or 0.5 points on Friday and made its way into the gainers of the day. After trading began at $10.66 the stock was seen hitting $11.88 as a peak level and $10.66 as the lowest level. The stock ended up at $11.55. The daily volume was measured at 1,072,987 shares. The 52-week high of the share price is $18.8999 and the 52-week low is $8.095. The company has a market cap of $580 million. Rockwell Medical, Inc. is up 9.9% in the last 3-month period. Year-to-Date the stock performance stands at 12.35%. The company shares have rallied 24.06% in the past 52 Weeks. On July 17, 2015 The shares registered one year high of $18.9 and one year low was seen on December 15, 2014 at $8.1. The 50-day moving average is $14.9 and the 200 day moving average is recorded at $12.18. S&P 500 has rallied 1.35% during the last 52-weeks.
On a different note, The Company has disclosed insider buying and selling activities to the Securities Exchange, According to the information disclosed by the Securities and Exchange Commission in a Form 4 filing, the CEO of Rockwell Medical, Inc., Chioini Robert L, had purchased 4,910 shares in a transaction dated on August 14, 2015. The transaction was executed at $11.96 per share with total amount equaling $58,724. Rockwell Medical, Inc. (NASDAQ:RMTI) stock has received a short term price target of $ 15 from 6 Analyst. The share price can be expected to fluctuate from the mean short term target, can be seen from the standard deviation reading of $8.15. The higher estimate of target price is $26 , while the lower price target estimate is $4 Rockwell Medical, Inc., formerly Rockwell Medical Technologies, Inc., manufactures hemodialysis concentrate solutions and dialysis kits, and it sells, distributes and delivers these and other ancillary hemodialysis products primarily to hemodialysis providers in the United States, as well as internationally primarily in Asia, Latin America and Europe. Hemodialysis duplicates kidney function in patients with failing kidneys also known as End Stage Renal Disease (ESRD). ESRD is an advanced-stage of chronic kidney disease (CKD) characterized by the irreversible loss of kidney function. Its dialysis solutions (also known as dialysate) are used to maintain life, removing toxins and replacing nutrients in the dialysis patients bloodstream. As of December 31, 2011, it was licensed and was developing renal drug therapies. During the year ended December 31, 2011, it acquired an abbreviated new drug application (ANDA) for a generic version of an intravenous Vitamin-D analogue, calcitriol.
|
|
Acetaminophen Boosts CGM Glucose Readings - Renal and Urology News |
 |
 |
August 24, 2015
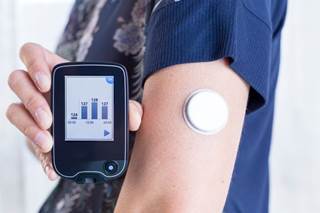
CGM glucose values increased versus blood glucose meter values in acetaminophen challenge.
(HealthDay News) -- Acetaminophen falsely increases continuous glucose monitor (CGM) glucose values, according to an observation letter published in Diabetes Care.
David M. Maahs, M.D., Ph.D., from the University of Colorado in Aurora, and colleagues examined the magnitude of the effect of acetaminophen on CGM glucose, particularly in the outpatient setting with contemporary sensor technology. The authors examined the potential challenges to closed-loop systems by performing the acetaminophen challenge in 40 individuals with hemoglobin A1c 7.3 ± 0.8%. Participants ingested 1,000 mg acetaminophen and obtained blood glucose (BG) meter readings over 8 hours after ingestion.
The researchers found that for 8 hours after acetaminophen ingestion there were significant differences (P ? 0.01 for all), consistent with expected pharmacokinetics. The greatest mean difference was 61 mg/dL. There was considerable individual variation; over 8 hours, 50% of relative differences were within 20% and an additional 26% ere within 40%. Three subjects had a BG meter value of <70 mg/dL with higher CGM readings, while 10 had a CGM >180 mg/dL and a BG meter value that was lower by more than 100 mg/dL.
"These data have implications for use of CGM glucose as a replacement for meter glucose for diabetes decision making and for closed-loop systems using CGM glucose values for automated insulin delivery," the authors write.
Several authors disclosed financial ties to the pharmaceutical and medical device industries.
Source
- Maahs, DM; DeSalvo, D; Pyle, L; et al. Diabetes Care published online August 12, 2015; doi: 10.2337/dc15-1096.
|
|
|
Ardelyx (ARDX) - Analysts' Weekly Ratings Changes - Dakota Financial News |
 |
 |
|
 Several brokerages have updated their recommendations and price targets on shares of Ardelyx (NASDAQ: ARDX) in the last few weeks: Several brokerages have updated their recommendations and price targets on shares of Ardelyx (NASDAQ: ARDX) in the last few weeks:
- 8/20/2015 – Ardelyx was downgraded by analysts at Zacks from a “strong-buy” rating to a “hold” rating. According to Zacks, “Ardelyx, Inc. is a clinical-stage biopharmaceutical company. It discovers, develops and commercializes small molecule therapeutics that work in the gastrointestinal tract to treat cardio-renal, GI and metabolic diseases. The Company’s lead product candidate is Tenapanor which is in three ongoing Phase II clinical trials for the treatment of patients with ESRD-HD and chronic kidney disease, as well as for constipation-predominant irritable bowel syndrome. Ardelyx, Inc. is headquartered in Fremont, California. “
- 8/19/2015 – Ardelyx had its “buy” rating reaffirmed by analysts at Wedbush.
- 8/18/2015 – Ardelyx had its price target raised by analysts at Wedbush from $19.00 to $24.00. They now have an “outperform” rating on the stock.
- 8/4/2015 – Ardelyx was upgraded by analysts at Zacks from a “strong sell” rating to a “buy” rating. They now have a $22.00 price target on the stock. According to Zacks, “Ardelyx, Inc. is a clinical-stage biopharmaceutical company. It discovers, develops and commercializes small molecule therapeutics that work in the gastrointestinal tract to treat cardio-renal, GI and metabolic diseases. The Company’s lead product candidate is Tenapanor which is in three ongoing Phase II clinical trials for the treatment of patients with ESRD-HD and chronic kidney disease, as well as for constipation-predominant irritable bowel syndrome. Ardelyx, Inc. is headquartered in Fremont, California. “
Ardelyx Inc (NASDAQ:ARDX) opened at 17.41 on Monday. The firm has a market capitalization of $451.34 million and a price-to-earnings ratio of 146.30. Ardelyx Inc has a 12 month low of $7.95 and a 12 month high of $35.48. The company has a 50 day moving average price of $18.12 and a 200 day moving average price of $14.92.
Ardelyx (NASDAQ:ARDX) last issued its quarterly earnings results on Wednesday, August 12th. The biopharmaceutical company reported $0.42 EPS for the quarter, topping the Zacks’ consensus estimate of $0.09 by $0.33. The business had revenue of $17.70 million for the quarter, compared to the consensus estimate of $25.87 million. Analysts anticipate that Ardelyx Inc will post ($1.11) earnings per share for the current fiscal year.
In related news, VP Elizabeth A. Grammer sold 2,500 shares of the stock in a transaction dated Wednesday, July 22nd. The stock was sold at an average price of $18.00, for a total transaction of $45,000.00. The sale was disclosed in a legal filing with the Securities & Exchange Commission, which can be accessed through this link. Also, VP Elizabeth A. Grammer sold 10,000 shares of the stock in a transaction dated Wednesday, July 15th. The shares were sold at an average price of $17.12, for a total value of $171,200.00. The disclosure for this sale can be found here.
Ardelyx, Inc. is a clinical-stage biopharmaceutical company focused on the discovery, development and commercialization of minimally-systemic, small molecule therapeutics that work exclusively in the gastrointestinal (NASDAQ:ARDX) tract to treat cardio-renal, GI and metabolic diseases. The Company utilizing its platform, discovered and designed its lead product candidate, tenapanor, which in clinical studies has demonstrated the ability to improve the symptoms of constipation-predominant irritable bowel syndrome (IBS-C) and to reduce the absorption of both dietary sodium and phosphorus. The Company in collaboration with AstraZeneca, has completed a Phase IIb clinical trial evaluating tenapanor in patients with IBS-C. It also has other product candidates under development, such as RDX002 for the treatment of Hyperphosphatemia, RDX009 for the treatment of IBD, Short Bowel Syndrome and non-alcoholic steatohepatitis (NASH), and RDX013 for the treatment of hyperkalemia.
Receive News & Ratings for Ardelyx Inc Daily - Enter your email address below to receive a concise daily summary of the latest news and analysts' ratings for Ardelyx Inc and related companies with MarketBeat.com's FREE daily email newsletter.
|
|
DaVita (DVA) to Acquire Renal Ventures in $415M Deal - StreetInsider.com |
 |
 |
|
DaVita (NYSE: DVA) has entered into an agreement to acquire Colorado-based Renal Ventures Limited, LLC ('Renal Ventures') – including a 100 percent interest in all dialysis centers owned by Renal Ventures – for $415 million.
Javier Rodriguez, CEO of DaVita Kidney Care said, "We are excited to work with Renal Ventures' patients, employees and physicians. Our commitment is, and will always be about enhancing the quality of life for our patients. We look forward to serving Renal Ventures' patients with industry-leading outcomes and comprehensive care."
Renal Ventures operates 36 dialysis clinics in six states. Multispecialty Physician Partners and Physician Venture Partners, divisions of Renal Ventures, operate infusion and vascular centers, respectively, in three states.
"Renal Ventures takes great pride in its culture of actively engaging our employees, patients and the communities we serve. The quality of care delivered by our caregivers, along with recognition as 'Best Place to Work,' are a source of pride," said Larry Chatfield, CEO of Renal Ventures. "In today's changing health care environment, combining with DaVita, a patient-centric organization, will benefit all of our stakeholders."
DaVita operates 2,210 clinics in 46 states and is the demonstrated clinical leader according to two recent government reports, the CMS Five-Star Rating System and the CMS Quality Incentive Program.
Credit Suisse acted as financial advisor to DaVita on this transaction. Morrison & Foerster LLP served as lead counsel, and Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C., served as regulatory counsel to DaVita.
For Renal Ventures, Brownstein Hyatt Farber Schreck, LLP served as lead counsel and Raymond James and Associates served as financial advisor.
|
|
 Log in to explore the world's most comprehensive database of dialysis centres for free!
Log in to explore the world's most comprehensive database of dialysis centres for free!  Professional dialysis recruitment
Professional dialysis recruitment

 TOP STORIES
TOP STORIES
 Several brokerages have updated their recommendations and price targets on shares of Ardelyx (NASDAQ: ARDX) in the last few weeks:
Several brokerages have updated their recommendations and price targets on shares of Ardelyx (NASDAQ: ARDX) in the last few weeks:
